Introduction: (Initial Observation)
Milk is a nutritious food with special physical, chemical and biochemical properties. Products made from milk are generally known as dairy products; however, milk is also used in production of some chemicals including plastics, adhesives and paints.
As we learn about the milk properties we may come up with new ideas and find new uses for milk. We may also find answers to some of the questions that we may have about the milk.

In one experiment with the milk, drops of food coloring are placed in milk, then detergent is added. That results a dramatic interactions between molecules at the surface of the milk. Explanation and determining the cause of such phenomenon is the main subject of this project.
Some additional questions about milk are:
- Why do we keep milk in a refrigerator? Can we simply add some preservatives or antibiotics to prevent milk from getting spoiled?
- How can we make plastics from milk? Can we use spoiled milk to make plastic?
- What are the ingredients of the milk?
- Why is milk white? Which of the milk ingredients contribute to its white color?
- What percent of milk is water?
- How dry milk is made?
Each of the above questions and thousands of similar questions can be the subject of a different science project involving milk.
Information Gathering:
Find out about milk, its composition, properties and applications. Read books, magazines or ask professionals who might know in order to learn about the production of different milk products. Keep track of where you got your information from.
Milk is a more complex substance than a simple solution. It contains not only a variety of salts and sugars dissolved in water, but also small globules of fatty substances (cream) and protein which vary in diameter. The more recent the homogenizing process, the smaller the emulsified fat globules. The fat globules, being hydrophobic, cannot dissolve in the water. They can however dissolve into each other.
When learning about the hydrophilic and hydrophobic components of milk, also review the general structure of a detergent molecule. The structure of a detergent molecule includes the hydrophilic and hydrophobic ends. The structural formula of detergent is shown below:
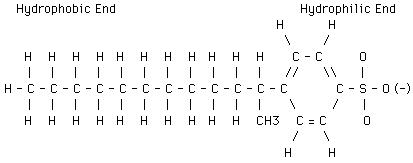
A description of the average composition of bovine milk is given below:
| Substance | Avg. % composition |
| water | 87% |
| total solids | 13% |
| a) proteins (mostly casein) | 3.0 – 4.0% |
| b) lipids (mostly triglycerides) | 3.5 – 5.0% |
| c) sugars (primarily lactose) | 4.5 – 5.0% |
Question/ Purpose:
What do you want to find out? Write a statement that describes what you want to do. Use your observations and questions to write the statement.
The purpose of this project is to observe and explain the unusual behavior of milk as it is acted upon by a common household detergent.
Why a project about milk? State the problem.
The problem is that milk will easily spoil and becomes unusable or wasted. Producers of milk who handle very large amounts of milk every day are really concerned about wasting milk as their main product. They want to know it better so they can preserve it or they can convert it to other valuable products.
Identify Variables:
When you think you know what variables may be involved, think about ways to change one at a time. If you change more than one at a time, you will not know what variable is causing your observation. Sometimes variables are linked and work together to cause something. At first, try to choose variables that you think act independently of each other.
Independent variables are:
- Water contents (or solid contents of the milk)
- Percent of fat in the milk
- Age of the milk (time from homogenization)
Dependent variable is the interaction of detergent with milk. (none, slow, fast)
controlled variables are the source of milk, temperature and experiment procedures.
Hypothesis:
Based on your gathered information, make an educated guess about what types of things affect the system you are working with. Identifying variables is necessary before you can make a hypothesis.
Following are two possible hypothesis:
- A drop of detergent reduces the surface tension of the milk at its point of contact, so all other surface molecules that are still subject to a tension will be pulled toward the walls of the dish.
- A drop of detergent tends to distribute on the surface of the milk, so it pushes back other surface molecules including food coloring.
Experiment Design:
Design an experiment to test each hypothesis. Make a step-by-step list of what you will do to answer each question. This list is called an experimental procedure. For an experiment to give answers you can trust, it must have a “control.” A control is an additional experimental trial or run. It is a separate experiment, done exactly like the others. The only difference is that no experimental variables are changed. A control is a neutral “reference point” for comparison that allows you to see what changing a variable does by comparing it to not changing anything. Dependable controls are sometimes very hard to develop. They can be the hardest part of a project. Without a control you cannot be sure that changing the variable causes your observations. A series of experiments that includes a control is called a “controlled experiment.”
You will design your own experiments. Each experiment must have a purpose. Experiment 1 that I have suggested below is just the start point for you to see how a touch of detergent can push away the color spots. In order to determine the cause, you will need to design and perform many similar experiments. The results of your experiments will be used to draw a conclusion. You may modify the variables or substitute milk with water or food colors with wood dust as long as you have an idea and you know how you want to use the results in your conclusion. You may even change the order of doing things. For example in one experiment you may first touch the milk surface with detergent tooth pick ad then place color spots.
Experiment 1: How do detergents affect milk?
Small amounts of detergents may affect the chemical or physical properties of milk. In this experiment you display or observe how do detergents interact with milk.
Procedure:
Pour the milk into a small aluminum pie pan or a petri dish to a depth of about one cm. The milk should have time to “sit” for a minute so that there aren’t any currents from the pouring process. A couple of drops of the four different water soluble food colorings should then be added to the milk near the edge of the container at 0, 90, 180, and 270 degrees.
Observations may begin when a toothpick dipped in detergent is touched to the surface of the milk in the center of the pie pan. You may repeat this experiment as many times as you wish in order to enhance your observations.
Do you have any explanation for the phenomena you observed? How is it possible that the fairly quiet pan of milk is now exhibiting such activity? Propose a hypothesis to explain this unusual activity.
Variations
In order to determine the cause of such interaction and test your hypothesis, repeat your experiment with different values of independent variables that you have selected.
It is important that you use your current beliefs about milk, or aqueous mixtures in general, to think of possible explanations for the observed behavior. This process leads naturally to the development of controlled experiments to test your hypothesis. The phenomenon appears to be related to the detergent action on the surface tension of the milk. You may repeat variations of this experiment by:
- Varying the fat content of the milk (Try 0% fat, low fat, …)
- Varying the age of the milk (time from homogenization)
- Using various dilutions of the milk (skim, 2%, whole).
When finished, simply pour the used milk down the drain and wash the pans or petri dishes with a little detergent. Rinse the milk down the drain with plenty of water to prevent a sour smell in days to come.
Experiment 2: (Plastic from milk)
Casein plastics were introduced at the beginning of the 20th century, their starting material being the protein in cows milk, precipitated by the action of the enzyme rennin.
Although casein is readily molded to shape under moderate heat and pressure, it does not produce a stable material for manufacture until it has become hardened by soaking in formalin (5% solution of formaldehyde in water) for a long period. Unfortunately, this causes much distortion so casein plastics are almost always produced by machining stock material such as sheet, rod, tube or button blanks (small discs). After machining, casein may be polished either mechanically with abrasives or chemically with a ‘dip polish’.
In this experiment you make plastic from milk. In other words you extract casein from milk.
Materials:
- a pot or saucepan
- two cups milk
- two tablespoons of vinegar or lemon juice
Procedure:
- Place milk in saucepan and warm on stove to skin temperature (about 37°:C).
- Add vinegar or lemon juice while stirring. The milk will quickly coagulate.
- Roll out mass on a sheet of aluminum foil or a cutting board. Drain excess liquid.
- Let the resulting “plastic” sheet dry overnight.
What is happening? (Experiment Results)
The globules in milk are a combination of fat and protein. The protein is bound up in tight coils, through internal “bonding” – electronic interactions between adjacent atoms. A way to think of this is to visualize the protein as a caterpillar curled up into a ball and holding on to its own legs. Rolled up like this, the caterpillar wouldn’t interact with any other caterpillars or pretty much anything else.
Vinegar and lemon juice are both mild acids (they contain acetic acid and citric acid, respectively) and they are capable of “denaturing” the proteins in the milk globules. What this means is that they are capable of prying the molecules open and breaking some of the internal bonds that hold the atoms to each other. Effectively, they are capable of “unrolling” the caterpillar and exposing its legs.
The soft interior part of the protein is very “sticky” in a molecular sort of way. That is, it still wants to bond to other molecules and so it grabs the nearest neighboring site and holds on. The result is a higgledy-piggledy mess of proteins holding onto one another – a bit like the balls of caterpillars that sometimes one finds in trees. Everyone is holding onto as many others as possible.
Individually, the balls of protein in milk slide past one another without noticing. But joined into one big mess, they quickly coagulate to form a type of natural polymer. It is edible – although not particularly pleasant tasting if it is made with vinegar. It is the basis for making cheese although there are some additional steps in the cheese making process. And this type of polymer has been used in the past to make such things as buttons.
By letting it dry overnight, the excess water is removed by evaporation and a very firm plastic is formed. It can be colored and shaped, but this is more easily accomplished prior to drying when the protein is still quite wet.
Of course, this experiment can be scaled up or down very easily. Oh, and the results are edible but not particularly pleasant tasting.
Materials and Equipment:
List of material may vary based on your final experiment design. Following is a sample:
- four – six sets of various food colorings
- One quart of milk
- One small container of household detergent (dishwashing liquid or shampoo)
- Materials for the Group
- One toothpick
- One pie pan or petri dish
Results of Experiment 1 (Observation):
Experiments are often done in series. A series of experiments can be done by changing one variable a different amount each time. A series of experiments is made up of separate experimental “runs.” During each run you make a measurement of how much the variable affected the system under study. For each run, a different amount of change in the variable is used. This produces a different amount of response in the system. You measure this response, or record data, in a table for this purpose. This is considered “raw data” since it has not been processed or interpreted yet. When raw data gets processed mathematically, for example, it becomes results. Following is a sample:
Prior to adding the dishwashing liquid, the food coloring stays together in “splotches” of color. This is in contrast to water, where the food coloring quickly dissipates throughout the liquid (this is a good experiment to run at the same time!). The reason behind this is that milk is not a simple liquid like water, but a colloidal suspension of fat and protein molecules. Of course, that really doesn’t explain things – unless you know what a colloidal suspension is!

A colloid occurs when microscopic particles of one substance become suspended in a second substance. Typically, these are spherical particles 100 to 10,000 atoms in diameter. Other examples include smoke and fog, which are colloids occurring in air. A colloidal suspension occurs when the particles are supported by the second substance and are not allowed to “drop out” of solution. Homogenized milk consists of tiny particles of fat and protein that are suspended in water. These particles will not naturally agglomerate but, instead, float in solution. They are kept aloft by the bouncing forces of other atoms. Hence, milk is a mixture of particles and liquid.
The food coloring preferentially dyes the fat – the color pigments are more soluble in the fat than water. The reason for this is the nature of the molecular interactions. Essentially, the coloring agents are more similar to the fat molecules than they are to the water molecules and therefore, since “like attracts like”, they color the fat.

The result is that the food coloring makes billions and billions of colored balls, suspended in the liquid portion of the milk. They move very slowly on their own which is why the coloring doesn’t spread throughout the liquid in the same way that it would in water.
The final step in the process – the addition of the dishwashing liquid – starts the flow of liquid. This is because the molecules in soap are unique. They contain an end that is very water soluble – called the “hydrophilic” head group – and a portion that really doesn’t like water – called the “hydrophobic” tail. Addition of a “pile” of dishwashing liquid in the middle of the dish results in the soap molecules “running away” to the edge of the dish. And because of their hydrophilic and hydrophobic portions, these soap molecules skate across the surface of the milk. They do not dissolve in it.
The result is the outward rushing effect that is seen immediately upon the addition of the dishwashing soap. This is followed by the soap reaching the edge where it begins to combine with the fat in the milk and gets “subducted” – drawn under the surface. As it goes, the soap drags with it the colored “fat balls” created by the food coloring. This rush across the surface and are forced under at the edge. They then travel through the solution to re-emerge in the heart of the pie plate as natural convection or mixing begins.
Since each fat molecule is individually colored and acts like a little ball, they get mixed up with other balls of color. The result is the myriad of unusual colors that emerge. All sorts of colors are available because all sorts of different mixtures of the four food coloring pigments are created. It is much like a pointillism painting – each dot is a single color but the whole is something quite different. This, coincidently, is also how T.V. sets and computer monitors come up with all of their variety of colors.
HYDROPHILIC: “water-loving”. The portion of a molecule or a whole molecule that is very soluble in water.
HYDROPHOBIC: “water fearing”. The portion of a molecule or a whole molecule that is not soluble in water.
Calculations:
No calculation is required for this project.
Summary of Results:
Summarize what happened. This can be in the form of a table of processed numerical data, or graphs. It could also be a written statement of what occurred during experiments.
It is from calculations using recorded data that tables and graphs are made. Studying tables and graphs, we can see trends that tell us how different variables cause our observations. Based on these trends, we can draw conclusions about the system under study. These conclusions help us confirm or deny our original hypothesis. Often, mathematical equations can be made from graphs. These equations allow us to predict how a change will affect the system without the need to do additional experiments. Advanced levels of experimental science rely heavily on graphical and mathematical analysis of data. At this level, science becomes even more interesting and powerful.
Conclusion:
Using the trends in your experimental data and your experimental observations, try to answer your original questions. Is your hypothesis correct? Now is the time to pull together what happened, and assess the experiments you did.
Why do color spots move away from the detergent spot? How did you reach to this conclusion. Include your discussion.
Related Questions & Answers:
What you have learned may allow you to answer other questions. Many questions are related. Several new questions may have occurred to you while doing experiments. You may now be able to understand or verify things that you discovered when gathering information for the project. Questions lead to more questions, which lead to additional hypothesis that need to be tested.
Possible Errors:
If you did not observe anything different than what happened with your control, the variable you changed may not affect the system you are investigating. If you did not observe a consistent, reproducible trend in your series of experimental runs there may be experimental errors affecting your results. The first thing to check is how you are making your measurements. Is the measurement method questionable or unreliable? Maybe you are reading a scale incorrectly, or maybe the measuring instrument is working erratically.
If you determine that experimental errors are influencing your results, carefully rethink the design of your experiments. Review each step of the procedure to find sources of potential errors. If possible, have a scientist review the procedure with you. Sometimes the designer of an experiment can miss the obvious.
References:
Visit your local library and find books related to milk, soaps, detergents, and emulsions. Such books are generally found in the chemistry, biology and food sections.
Submitted by John C. Hugo
White, A., P. Handler, and E. Smith, 1964. Principles of Biochemistry, Third Edition, New York: Mc Graw -Hill Book Company.



